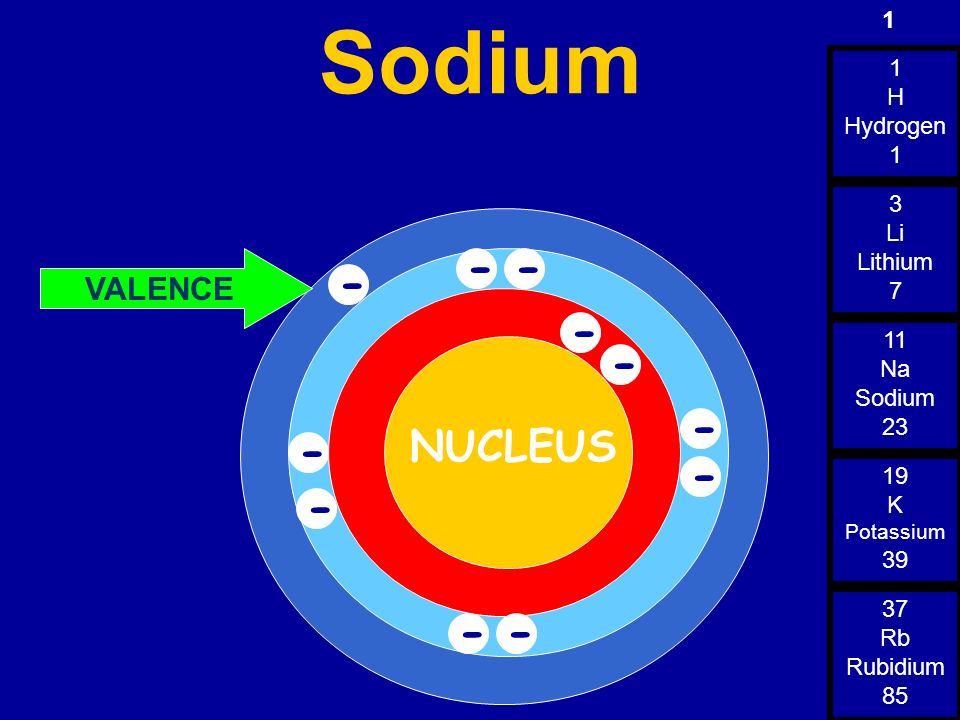
Correct set of four quantum numbers for the valence (outermost) electron of Rubidium (Z=37) is :
Rubidium Chloride (RbCl) Rubidium Floride (RbF) Rubidium Sulfate (Rb 2 SO 4) Interesting facts: It is the 16th most common element in the earth's crust. It is found in North America, Rush and South Africa. It can also be found in seawater and in mineral springs. It is not found freely in nature. Electronic configuration. Electronic configuration. 1s2 2s22p63s23p63ds24p65s1. Back to key information about the element.Sep 02, A step-by-step explanation of how to draw the Lewis dot structure for Rb (Rubidium). I show you where Rubidium is on the periodic table and how to determine how many valence electrons Rubidium.
(IIT JEE 1986)
1) 5, 0, 0, +½
2) 5, 1, 0, +½
3) 5, 1, 1, +½
4) 6, 0, 0, +½
Logic:
We may write the electronic configuration of Rubidium, Rb and then find out the quantum numbers for the last electron (Rb valence electron).
Or
Remember that, Rb belongs to alkali metals (1st group) and 5th period of modern periodic table. For alkali metals, the outer electronic configuration is ns1 and for the 5th period n = 5.
| Period | Elements of 1st group |
| 2 | 3Li |
| 3 | 11Na |
| 4 | 19K |
| 5 | 37Rb |
| 6 | 55Cs |
| 7 | 87Fr |
Solution:
Electronic configuration of Rb = 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1
Check that there are 37 electrons.
Therefore,
Principal quantum number, n = 5
Azimuthal quantum number, l = 0 (since for s orbital l = 0)
Magnetic quantum number, m = 0 (since for l = 0, there is only one m value i.e. 0)
Spin quantum number, s = +½ or -½ (there is only one electron and it can have clockwise or anticlockwise spins)
Conclusion:
The correct set of quantum numbers will be: 5, 0, 0, +½ or -½. Therefore correct option is 1.
SET OF QUANTUM NUMBERS
Followup questions
Q-1: The correct set of four quantum numbers for unpaired electron chlorine is :
1) 2, 0, +1, +½
2) 3, 0, -1, +½
3) 3, 1, -1, +½
4) 2, 0, 0, +½
Logic:
Write the outer electronic configuration of Chlorine atom.
It belongs to halogens i.e. 17th group and 3rd period of periodic table
Solution:
The outer electronic configuration of Chlorine atom in the ground state is: 3s2 3p5
The valence electron resides in one of the 3p orbital.
Hence the correct set of quantum numbers is: n=3, l=1, m=+1 or 0 or -1, s=+½ or -½.
Conclusion:
The correct set of quantum numbers among the given options is: 3, 1, -1, +½ i.e correct option is 3.
Q-2: The values of four quantum numbers of valence electron of an element are n=4, l=0, m=0 and s=+1/2. The element is :
(Eamcet - 2004-M)
a) K
b) Ti
c) Na
d) Sc
Logic:
n = 4 and l = 0 represent 4s orbital. Since it is valence electron, the element belongs to s-block and 4th period of periodic table.
Solution:
There are two elements that belong to s-block i.e. K and Na. On close inspection of periodic table, the quantum number data for the valence electron fits for potassium, K, since it belongs to 4th period.
Remember that the principal quantum number of valence electron represents the period number also that is especially true for s and p block elements.
Note: Na belongs to 3rd period and hence n should be 3 for the valence electron of this element. Ti and Sc belong to d-block.
Homework
1) Write all possible values of each quantum number for the outermost electron in an Rb atom.
2) What is the set of quantum numbers for unpaired electron of Fluorine atom?
3) Write the set of quantum numbers for the electron present in the first excited state of hydrogen atom.
4) How many sublevels are possible for n = 4 quantum level?
5) What is the principal quantum level of the highest energy electron of rubidium atom in the ground state?
$5,1,0,+frac{1}{2}$
C$5,1,1,+frac{1}{2}$
Rubidium Valence Electron Configuration
D$5,0,1,+frac{1}{2}$
Solution:
Given, atomic number of Rb, Z = 37 Thus, its electronic configuration is [Kr]5$s^1$. Since, the last electron or valence electron enter in 5s subshell.So, the quantum numbers are n = 5, l = 0, (for s-orbital) m = 0($because$ m = + l to -l), s = + 1/2 or -1 / 2.
1. Which one of the following molecules is expected to exhibit diamagnetic behaviour?
2. The rate of a reaction doubles when its temperature changes from $300, K$ to $310,K$. Activation energy of such a reaction will be $(R = 8.314 JK^{-1} mol^{-1} and log 2 = 0.301)$
3. Four successive members of the first row transition elements listed below with atomic numbers. Which one of them is expected to have the highest
$E_{m{^{3+}}/m{^{2+}}}^{circ}$value?
4. Consider the following reaction,
$xMnO_4^- + yC_2O_4^{2-}+zH^+ rightarrow xMn^{2+}+2yCO_2 + frac{z}{2}H_2O$
The values of x, y and z in the reaction are, respectively
5. Which of the following arrangements does not represent the correct order of the property stated against it?

6. A gaseous hydrocarbon gives upon combustion $0.72, g$ of water and $3.08, g$ of $CO_2$. The empirical formula of the hydrocarbon is
7. The order of stability of the following carbocations
How Many Valence Electrons Are In Rubidium
8. Experimentally it was found that a metal oxide has formula
$ M_{0.98}O. $ Metal $M$, present as $ M^{2+} $ and $ M^{3+} $
in its oxide.Fraction of the metal which exists as $ M^{3+} $
would be
9. Which of the following exists as covalent crystals in the solid state?
10. Arrange the following compounds in the order of decreasing acidity
1. The following nuclear transmission
$ce{^{23}_{11}Na + ^{1}_{1} H to ^{23}_{12} Mg + ^{1}_{0} n}$
belongs to
2. For emission line of atomic hydrogen from $n_i = 8$ to $n_f $ = the plot of wave number $(bar{v})$ against $( frac{1}{n^2})$ will be (The Ry dbergconstant, $R_H$ is in wave number unit).
3. Half-life of radium is 1580 years. Its average life will be
4. Which of the following arrangement is possible?
$quad$$quad$n$quad$ $quad$l$quad$$quad$m$quad$$quad$s
5. Quantum numbers of an atom can be defined on the basis of
6. Spectrum of Li2+ is similar to that of
7. Azimuthal quantum number defines
8. The quantum number m of a free gaseous atom is associated with
9. The isoelectronic pair is
10. For principle quantum number n = 4, the total number of orbitals having l = 3 is
1. In Wolff‐Kishner reduction, the carbonyl group of aldehydes and ketones is converted into
2. Identify compound X in the following sequence of reactions:
3. Identify a molecule which does not exist.
4. Identify the incorrect match.
Name IUPAC Official Name A Unnilunium i Mendelevium B Unniltrium ii Lawrencium C Unnilhexium iii Seaborgium D Unununnium iv Darmstadtium
| Name | IUPAC Official Name | ||
|---|---|---|---|
| A | Unnilunium | i | Mendelevium |
| B | Unniltrium | ii | Lawrencium |
| C | Unnilhexium | iii | Seaborgium |
| D | Unununnium | iv | Darmstadtium |
Rubidium Valence Electron Quantum Number
5. Reaction between acetone and methyl magnesium chloride followed by hydrolysis will give :
6. Identify the correct statements from the following:
(a) $CO_2(g)$ is used as refrigerant for ice-cream and frozen food.
(b) The structure of $C_{60}$ contains twelve six carbon rings and twenty five carbon rings.
(c) $ZSM-5$, a type of zeolite, is used to convert alcohols into gasoline.
(d) $CO$ is colorless and odourless gas.
What Are Valence Electrons Quizlet
7. Which of the following set of molecules will have zero dipole moment ?
8. On electrolysis of dil.sulphuric acid using Platinum (Pt) electrode, the product obtained at anode will be:
9. An element has a body centered cubic (bcc) structure with a cell edge of 288 pm. The atomic radiusis:
Rubidium # Of Valence Electrons
10. Find out the solubility of $Ni(OH)_2$ in 0.1 M NaOH. Given that the ionic product of $Ni(OH)_2$ is $2 times 10^{-15}$
